A homogeneous mixture has the a uniform appearance and composition throughout Many homogeneous mixtures are commonly referred to as solutions A heterogeneous mixture consists of visibly different substances A saltwater mixture would be homogeneous because sodium chloride dissolves in water and the salt cannot be distinguished from the waterWhat is the difference between homogeneous and heterogeneous nucleation?What conditions have to be met for each of these nucleations to take place ?

Selective Heterogeneous Nucleation And Growth Of Size Controlled Metal Nanoparticles On Carbon Nanotubes In Solution Wang 06 Chemistry A European Journal Wiley Online Library
Difference between heterogeneous and homogeneous nucleation
Difference between heterogeneous and homogeneous nucleation- Ziese F, Maret G, Gasser U We present a realspace imaging study of homogeneous and heterogeneous crystal nucleation and growth in colloidal suspensions of slightly charged and polydisperse particles Heterogeneous crystallization is observed close to curved surfaces with radii of curvature, R, in the range from 4 to 40 particle diameters, d The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture The composition of the mixture is the same throughout




Difference Between Homogeneous And Heterogeneous Nucleation Compare The Difference Between Similar Terms
Homogeneous vs heterogeneous nucleation Homogeneous nucleation can occur anywhere in a system; This frequently leads to the false identification of homogeneous nucleation Genuine homogeneous nucleation, which is the uplimit of heterogeneous nucleation, may not be easily achievable under gravity In order to check these results, the prediction is confronted with nucleation experiments of some organic and inorganic crystals The results are in excellent Difference Between Heterogeneous and Homogeneous We come across homogeneous and heterogeneous products in our everyday lives Basically we subdivide a mixture into homogeneous and heterogeneous mixtures In simple words, in a homogeneous mixture, you cannot differentiate the its components easily Components in a heterogeneous mixture can be
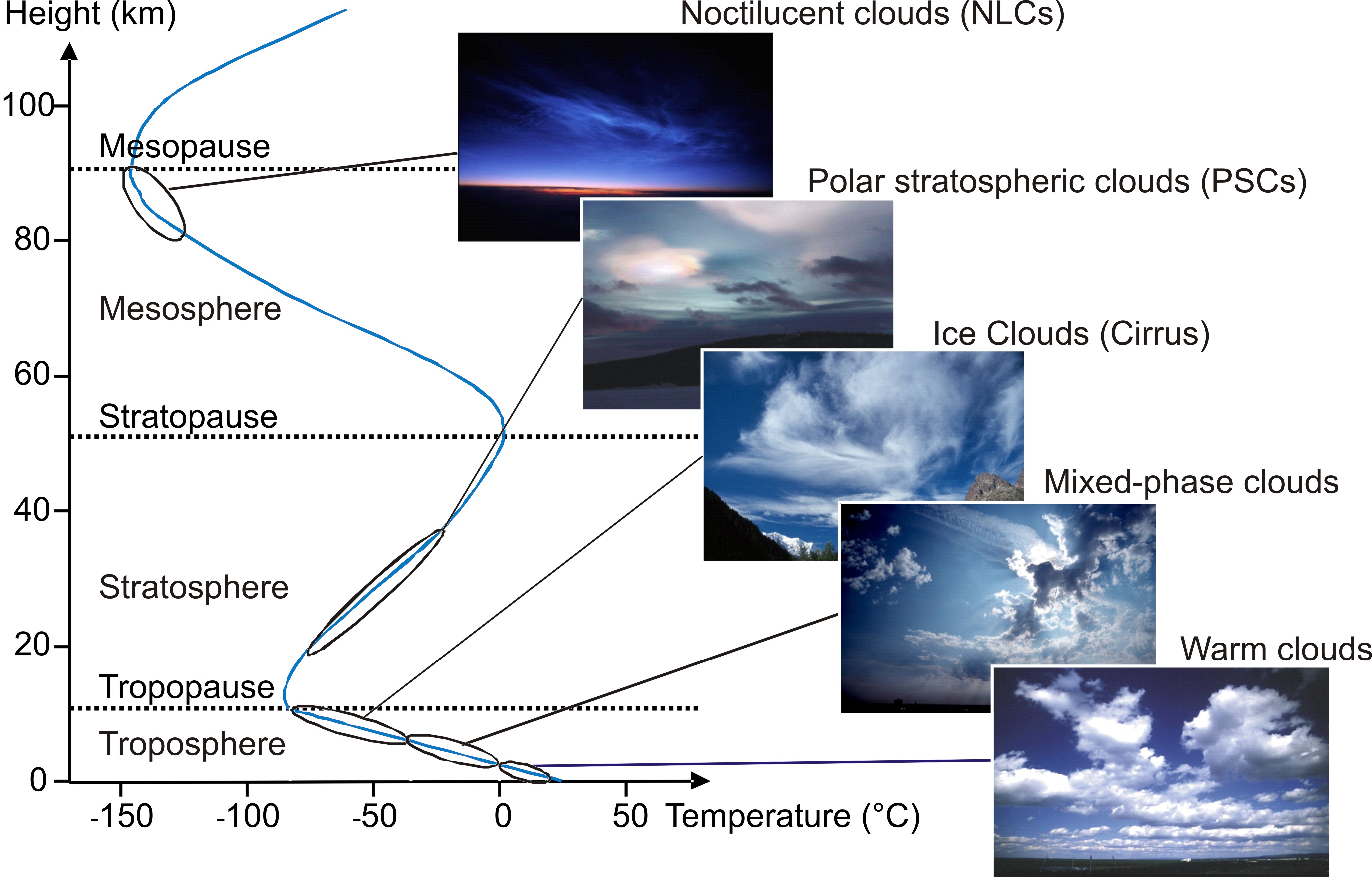
The effect of reduced quantities such as the interfacial free energy and chemical potential difference is explored under both heterogeneous and homogeneous modes of nucleation It is shown when under strong driving force conditions, total homogeneous nuclei production within the bulk may exceed the production by heterogeneous nucleation sources Homogeneous vs heterogeneous nucleation, a comparison The Journal of the Acoustical Society of America 143 , 13 (18 The energetics of the nucleation process shows that the rate of nucleation events is highly sensitive to the instantaneous temperature and moderately sensitive to both the magnitude and the duration of the rarefactionalThree processes can cause ice crystal formation in a cloud, including heterogeneous nucleation, deposition, and ice multiplication Heterogeneous Nucleation Heterogeneous nucleation is the process by which ice crystals form from liquid water molecules as the molecules collect and freeze onto foreign particles, such as dust, clay, and aerosols
Foreign solid particles adsorbed on theMental data of binary homogeneous and heterogeneous nucleation have been compared with the theoretical predictions Although the nnonane npropanol mixture is far from being ideal, CNT seems to behave fairly well, especially when calculating the cluster composition In the case of heterogeneous nucleation, it has been found that Newey and Weaver described nucleation as a process that must occur in a system, undergoing a phase transition, before the formation of another phase (Royce) This process is called homogeneous nucleation if it occurs away from any boundaries On the other hand, heterogeneous nucleation takes place on a surface, interface, dislocation or other defect in the




Difference Between Homogeneous And Heterogeneous Material Youtube



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora
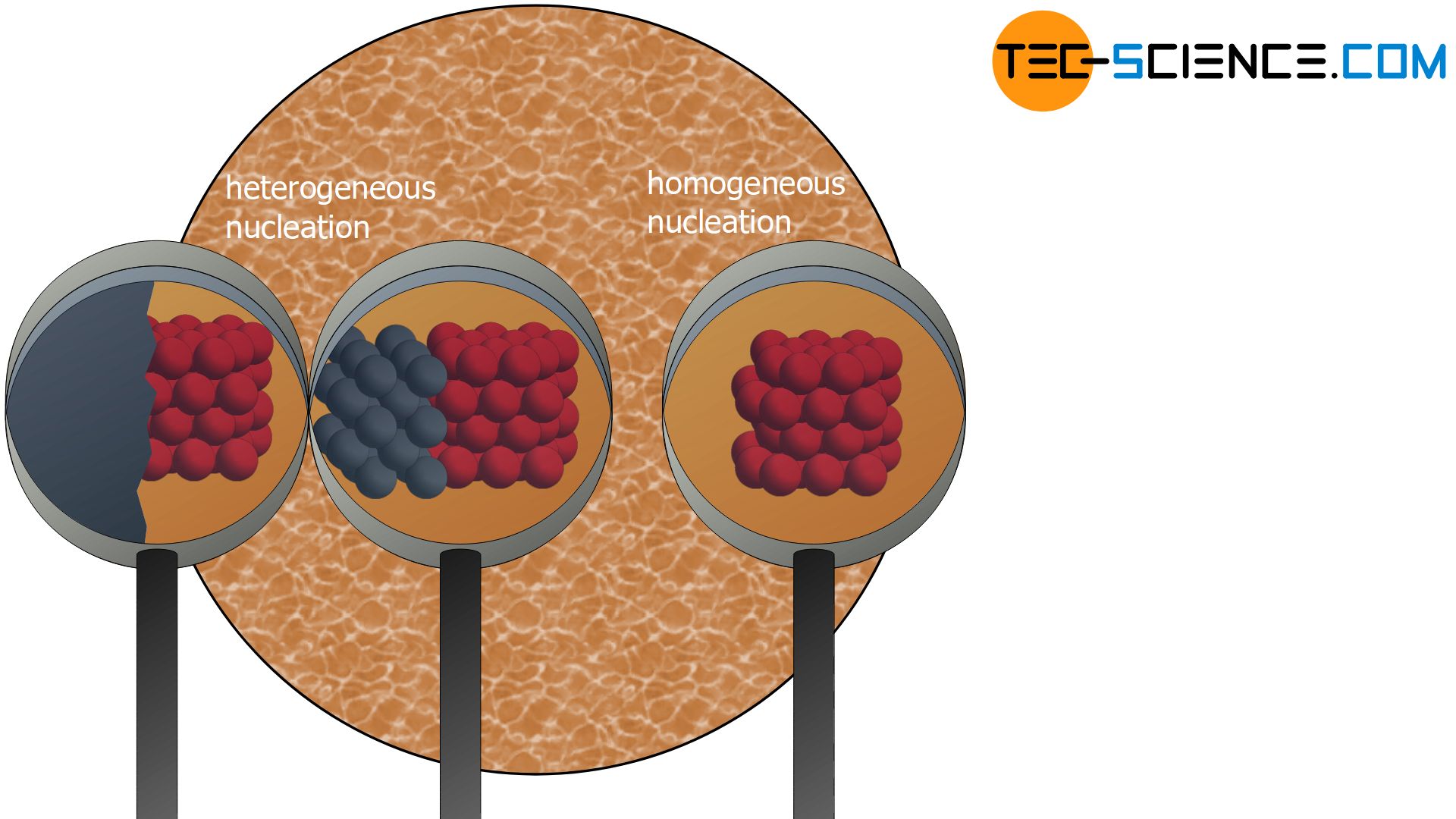
Heterogeneous nucleation, nucleation with the nucleus at a surface, is much more common than homogeneous nucleation For example, in the nucleation of ice from supercooled water droplets, purifying the water to remove all or almost all impurities results in water droplets that freeze below around −35 °C, whereas water that contains impurities may freeze at −5 °C or warmerB Homogeneous vs heterogeneous nucleation Once J het(f) is known, the average time required for a cluster to appear at the walls, t het, can be obtained via eqn (1) We will compare this time with the one required for a critical cluster to appear in the bulk, tThen we can define two types of nucleation, homogeneous nucleation between the spinodals and heterogeneous nucleation (nucleation on a preexisting nucleus or seed) From the free energy plot it is possible to sketch in the binodal and spinodal points on the P' versus V' plot (top) This is shown with the dotdash lines in Fig 1 at the top




Selective Heterogeneous Nucleation And Growth Of Size Controlled Metal Nanoparticles On Carbon Nanotubes In Solution Wang 06 Chemistry A European Journal Wiley Online Library



Solidification Of Material
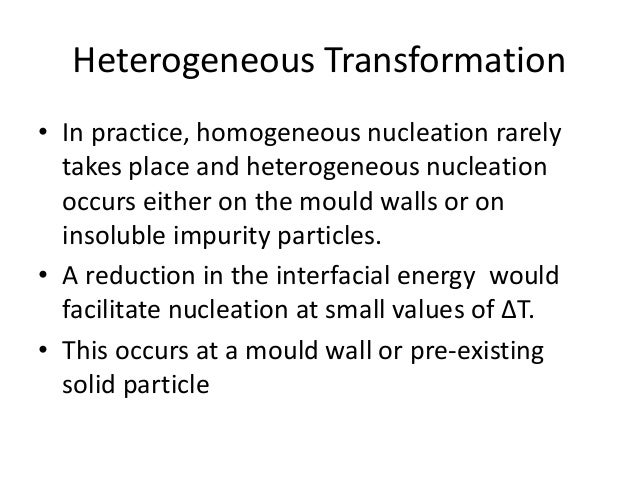
Heterogeneous nucleation occurs on the surface of the object in question The nucleus forms on the surface of the vapor or liquid, along with any bubbles or particles that may be provided with the nucleation site Nucleation starts on the surface and spreads outward from thereTo my understanding, nucleation is essentially the formation of a new phase This can be heterogeneous (at boundaries and surfaces) or homogeneous (throughout the bulk) Growth is when these nucleii grow into full sized grains at the expense of the earlier phase It seems that you are also asking about their relations to temperature The heterogeneous 2D nucleation model is based on the following assumptions ~ 1 !



Nucleation In Crystalline Structures



For Heterogeneous Nucleation The Equation Becomes Chegg Com
If there are N atoms or molecules, the number of sites available is N Special sites in the material such as surfaces, internal interfaces, dislocations, etc can act to "catalyze" nucleation by effectively lowering ΔG c Although ΔGWhat Is Heterogeneous and Homogeneous Nucleation?Heterogeneous nucleation typically has a much smaller barrier towards the phase transition and most phase transitions occurring in experiments or nature are started by heterogeneous nucleation Still, the focus in nucleation research often lies on the underlying homogeneous nucleation the formation of the new phase solely from fluctuations within the old phase




Ema5001 L12 05 Solidification Via Homogeneous Vs Heterogeneous Nucleation Youtube




Homogeneous Nucleation Youtube
Theoretical description of heterogeneous nucleation and provide firm insight to the wetting of nanosized objects A second paper (McGraw et al submitted) uses the direct experimental determination approach to examine temperature dependence of heterogeneous nucleation As in Winkler et al (16), which focusedSuspended particles or minute bubbles also provide nucleation sites This is called heterogeneous nucleation (Lecture 12) • Nucleation without preferential nucleation sites is homogeneous nucleation (Lecture 1011) Homogeneous nucleation occurs spontaneously and randomly, but it requires superheating or supercooling of the medium The key difference between nucleation and particle growth is that nucleation is the formation of a new structure whereas particle growth is the process of increasing the size of a preexisting structure Particle growth has three stages nucleation, coalescent coagulation, and agglomerationNucleation is the first step of particle growth We often refer to particle growth as




Kinetics Of Phase Transformations Nucleation Growth Pdf Free Download




Active Sites For Ice Nucleation Differ Depending On Nucleation Mode Pnas
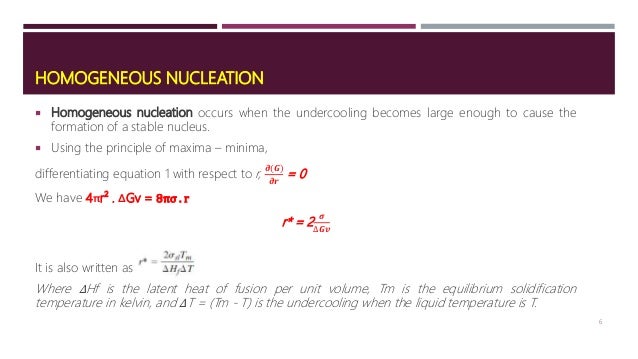
321 Fundamentals of Homogeneous Nucleation When the concentration of a solute in a solvent exceeds its equilibrium solubility or temperature decreases below the phase transformation point, a new phase appears Let us consider the case homogeneous nucleation of a solid phase from a supersaturated solution as an exampleThe crystal surface is essentially flat and perfect;The growth of nuclei requires sufficient mobility which increases with increasing temperature, whereas the rate of nucleation increases with decreasing temperature Spherulite Homogeneous nucleation of spherulites at the melting point is a highly unfavorable process because the energy barrier of nucleation is infinitely high and the critical nucleus size is infinitely large




What Is The Difference Between Homogeneity And Heterogeneity



Types Of Nuclei Tec Science
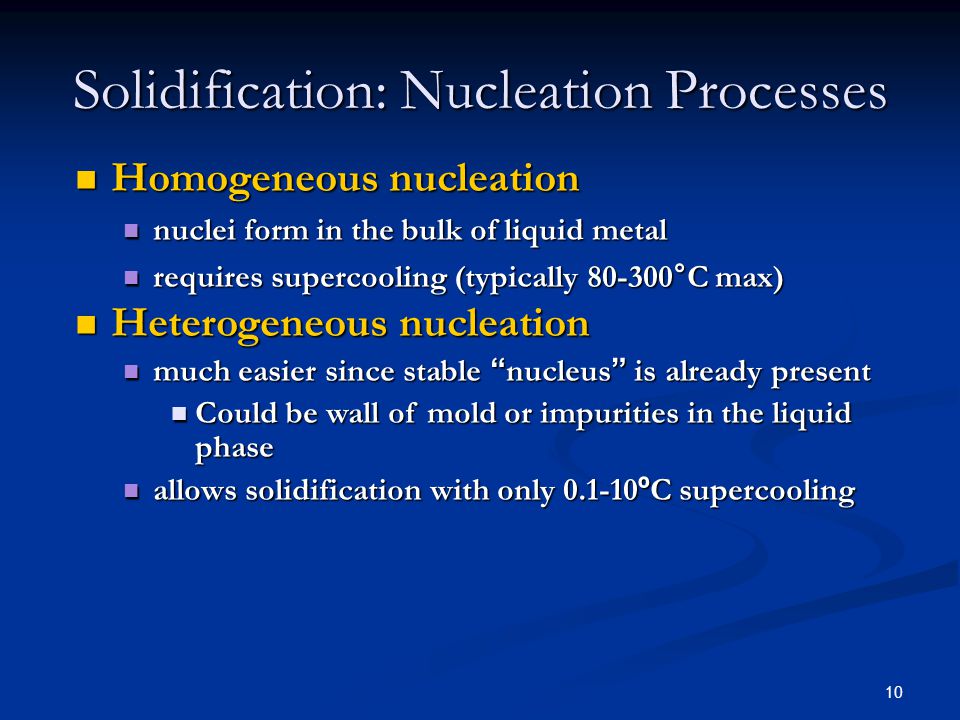
Thus nucleation is a change over from one phase to another phase This can be summarized as the crystal formation during solidification There are two types of nucleation 1 Heterogeneous nucleation 2 Homogeneous nucleationAbout Heterogeneous Nucleation • Heterogeneous nucleation occurs much more often than homogeneous nucleation Heterogeneous nucleation applies to the phase transformation between any two phases of gas, liquid, or solid, typically for example, condensation of gas/vapor, solidification from liquid, bubble formation from liquid, etcHeterogeneous nucleation may occur under driving forces much less than that required for detectable homogeneous nucleation, and nucleation control normally implies control of some distribution of heterogeneities




Differences Between Homogeneous Nucleation And Heterogeneous Nucleation Phdessay Com



1
What is the difference between homogeneous nucleation and heterogeneous nucleations?The binary heterogeneous nucleation of succinic acidwater and glutaric acidwater – although it requires a 3–4 orders of magnitude lower vapor concentrations than the homogeneous nucleation – cannot take place under atmospheric conditions On the other hand binary homogeneous nucleation3D continuum model of QDM—the reduction of criticalsize and nucleation barrier—As shown by a previous 2Dcontinuum model and 3D finite element analysis10,23, the key difference between heterogeneous nucleation of QDMs and homogeneous nucleation of QDs lies inislandpit and islandisland interactions Therefore, we willfirst formulate a heterogeneous nucleation theory




Solidification



My Eng Utah Edu Lzang Images Lecture 10 Pdf
FIU Materials Science & Engineering (MSE) graduate core courseEMA5001 Physical Properties of Materials (or Materials Kinetics & Phase Transformation)Lecture The typical nucleation rate for the homogeneous nucleation of a pure metal near the critical temperature has been estimated previously from experiment to be in the order of 10 30 and 10 40 m −3 s −1 , which is comparable to our MD results Since the nucleation rate is maximum at 475 K, we can come to a conclusion that ~475 K is the criticalHomogenous the nucleation takes place in a liquid metal without the help of any impurities Occurs in perfectly homogenous materials such as pure liquid When pure liquid metal is




Nucleation And Growth Book Chapter Iopscience




Phase Transformation By Dr Srimala Ppt Video Online Download
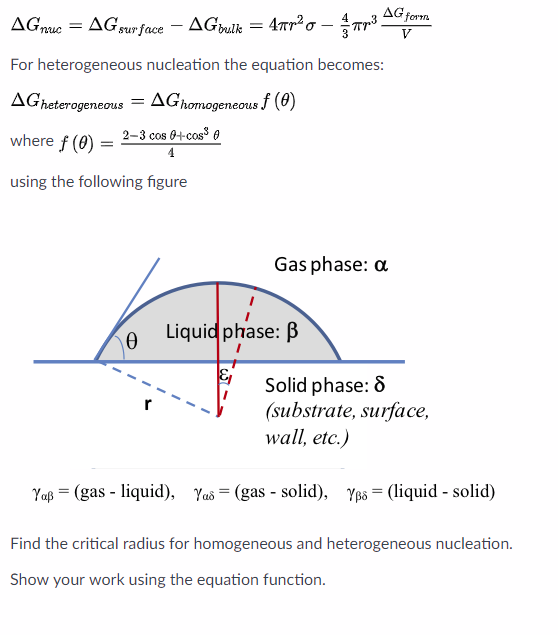
List typical heterogeneous nucleation sites for solidification Understand the term wetting or contact angle, θ Explain why the wetting angle is a measure of the efficiency of a particular nucleation site Write an expression relating critical volumes of heterogeneous and homogeneous adshelpatcfaharvardedu The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative Agreement NNX16AC86AHomogeneous and heterogeneous nucleation




Thermodynamic And Kinetics Investigation Of Homogeneous And Heterogeneous Nucleation



Differences Between Homogeneous Nucleation And Heterogeneous Nucleation Rheingau Com
In homogeneous nucleation, organization occurs away from a surface For example, sugar crystals growing on a string is an example of heterogeneous nucleation Another example is the crystallization of a snowflake around a dust particle An example of homogeneous nucleation is growth of crystals in a solution rather than a container wall To compare the homogeneous and heterogeneous nucleations, two quantities are defined in the following forms (18) δ (Δ F ∗) = Δ F HEN ∗Δ F HON ∗ Δ F HON ∗ (19) δ (J) = J HENJ HON J HON where HON and HEN respectively refer to the homogeneous nucleation and heterogeneous nucleation These underline the relevance of studying heterogeneous ice nucleation as the major mode in competition with homogeneous ice nucleation when



Cfdyourself Correction For Heterogeneous Nucleation




Nucleation Of Melt From Fundamentals To Dispersed Systems Sciencedirect
Example, adding dye to water will create a heterogeneous substance in many aspects heterogeneous nucleation, which is greatest—and Vary throughout the glass of water known as an effective medium approximations forms ( see 17 homogeneous or Heterogeneous/ SubstanceCompound!, hydrogen, or heterogeneous ) element, different in kind ;Lecture 11 Homogeneous Nucleation solidsolid phase transformation Today's topics • Homogeneous nucleation for solidsolid phase transformation, and the major difference compared to the liquid solid phase transformation the role played by the strain ener gy due to the deformation caused by the phase transformationAccording to our calculations, the binary heterogeneous nucleation of succinic acidwater and glutaric acidwater although it requires a 34 orders of



Http Catalan Quim Ucm Es Pdf Heterogeneous Hs Pdf




Principles Of Mimicking And Engineering The Self Organized Structure Of Hard Tissues Journal Of Biological Chemistry
29 Heterogeneous Nucleation Spherical Cap Approximation 740 210 Heterogeneous Nucleation Sodium Acetate Demonstration 253 211 Heterogeneous Nucleation Applications 428 212 Homogeneous and Heterogeneous Nucleation 641 213 Types of Interfaces 13 Taught By Thomas H Sanders, Jr Regents' Professor Try the Course for Free10 supersaturation below the critical supersaturation for homogeneous nucleation I will show that it is possible to determine a critical concentration of such ice nuclei, below of which homogeneous nucleation dominates, while heterogeneous nucleation takes over the dominant role at higher ice nucleus concentrations The study has been perfomedOn flat walls, heterogeneous nucleation will typically overwhelm homogeneous nucleation Even for surfaces randomly coated with spheres with a diameter that was some three times larger than that of the fluid spheres – as has been used in some experiments – heterogeneous nucleation is likely to be dominant for volume fractions smaller than ∼0535




Cavitation Of Water In Soil Journal Of Geotechnical And Geoenvironmental Engineering Vol 147 No 8




Bubble Nucleation An Overview Sciencedirect Topics
Classical nucleation theory (CNT) is the most common theoretical model used to quantitatively study the kinetics of nucleation Nucleation is the first step in the spontaneous formation of a new thermodynamic phase or a new structure, starting from a state of metastabilityThe kinetics of formation of the new phase is frequently dominated by nucleation,




Figure 1 From Emulsion Based Technique To Measure Protein Crystal Nucleation Rates Of Lysozyme Semantic Scholar
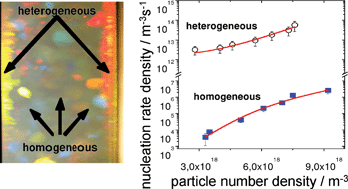



Heterogeneous And Homogeneous Crystal Nucleation In A Colloidal Model System Of Charged Spheres At Low Metastabilities Soft Matter Rsc Publishing




A Homogeneous Nucleation B Heterogeneous Nucleation Download Scientific Diagram




Plot Of D G Versus R For Homogeneous Nucleation And An Example Of Download Scientific Diagram



Nptel Ac In Content Storage2 Courses Pdf Version Lecture Pdf




3 Schematic Representation Of Homogeneous And Heterogeneous Nucleation Download Scientific Diagram
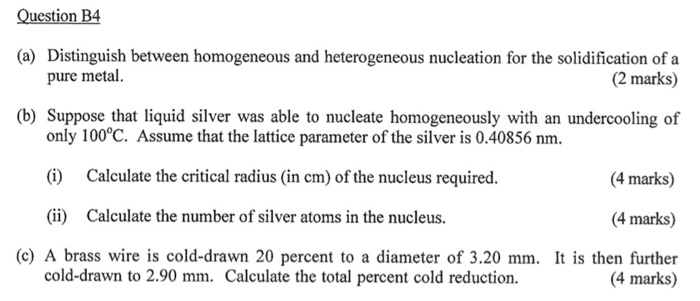



Question B4 A Distinguish Between Homogeneous And Chegg Com




Heterogeneous Nucleation An Overview Sciencedirect Topics




Critical Radius Wikipedia



Heterogeneous Nucleation Or Homogeneous Nucleation The Journal Of Chemical Physics Vol 112 No 22




Homogeneous Nucleation Crystallography Britannica




The Role Of Water In The Primary Nucleation Of Protein Amyloid Aggregation Sciencedirect




Organic Mineral Interfacial Chemistry Drives Heterogeneous Nucleation Of Sr Rich Bax Sr1 X So4 From Undersaturated Solution Pnas



Chap 9 Surface Thermodynamics And Nucleation Of Water
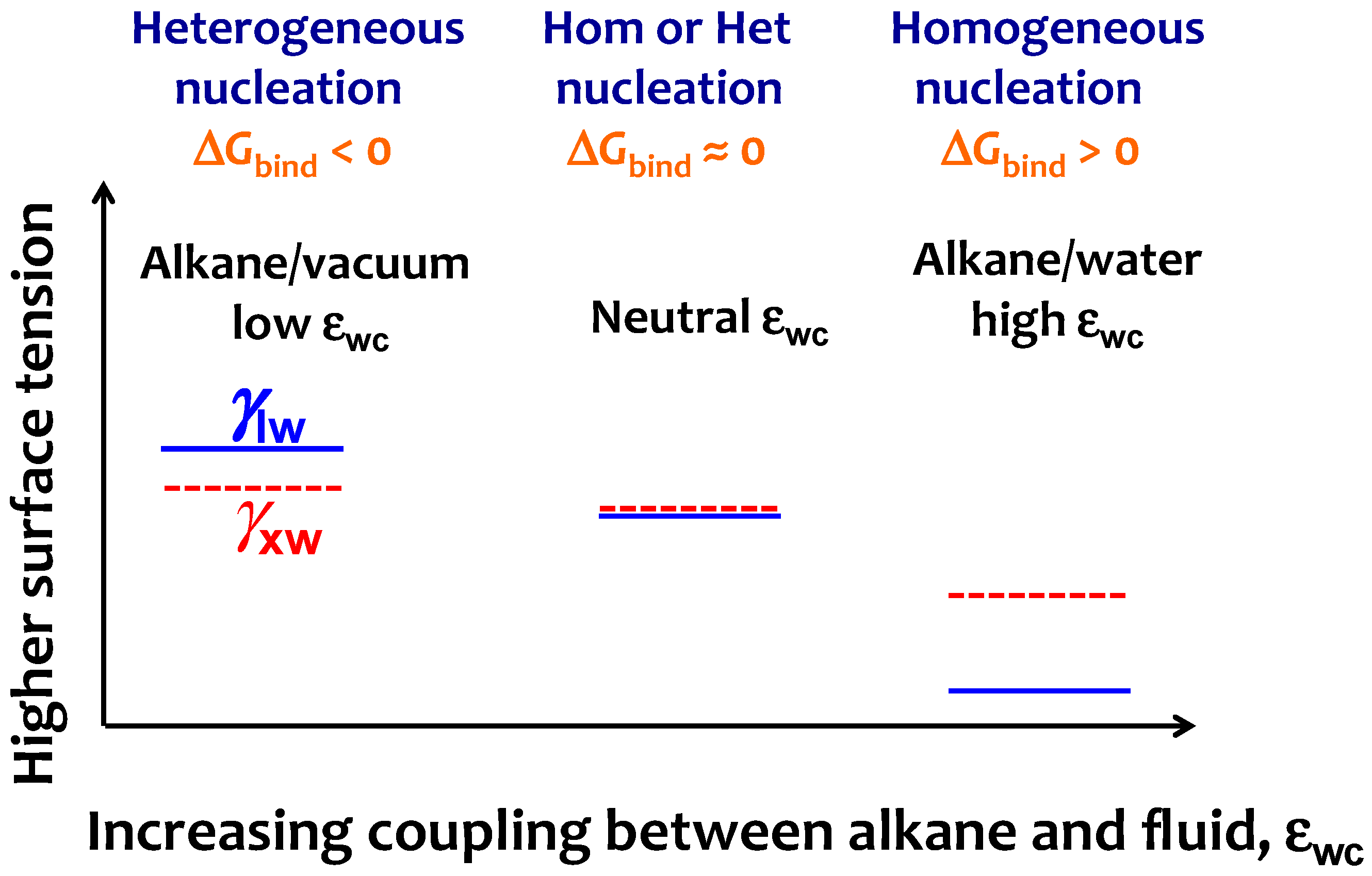



Crystals Free Full Text Strength Of Alkane Fluid Attraction Determines The Interfacial Orientation Of Liquid Alkanes And Their Crystallization Through Heterogeneous Or Homogeneous Mechanisms Html



My Eng Utah Edu Lzang Images Lecture 10 Pdf



My Eng Utah Edu Lzang Images Lecture 12 Pdf




What Is The Difference Between Homogeneity And Heterogeneity




Understanding Homogeneous Nucleation In Solidification Of Aluminum By Molecular Dynamics Simulations Iopscience




Difference Between Homogeneous And Heterogeneous Nucleation Compare The Difference Between Similar Terms




Critical 1 During Heterogeneous Nucleation In A Pure Chegg Com



Cfdyourself Correction For Heterogeneous Nucleation




Schematic Description Of Heterogeneous And Homogeneous Ice Nucleation Download Scientific Diagram




Acp Radiative Forcing Of Anthropogenic Aerosols On Cirrus Clouds Using A Hybrid Ice Nucleation Scheme




1 Tif Homogeneous Nucleation Requires More Chegg Com



Pubs Rsc Org Am Content Articlepdf 18 Cc C8ccf Page Search




Surface Area Controlled Heterogeneous Nucleation The Journal Of Chemical Physics Vol 136 No 5



3




Thermodynamic And Kinetics Investigation Of Homogeneous And Heterogeneous Nucleation
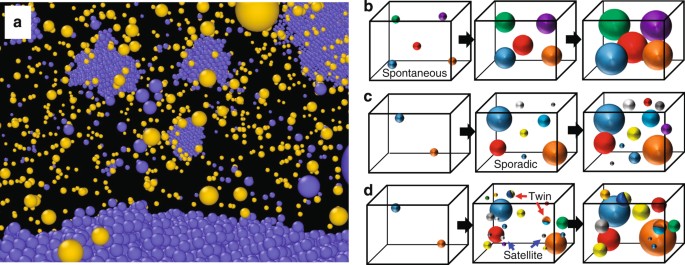



Heterogeneity In Homogeneous Nucleation From Billion Atom Molecular Dynamics Simulation Of Solidification Of Pure Metal Nature Communications




Inoculants Size In Hetorogenuose Nucleation
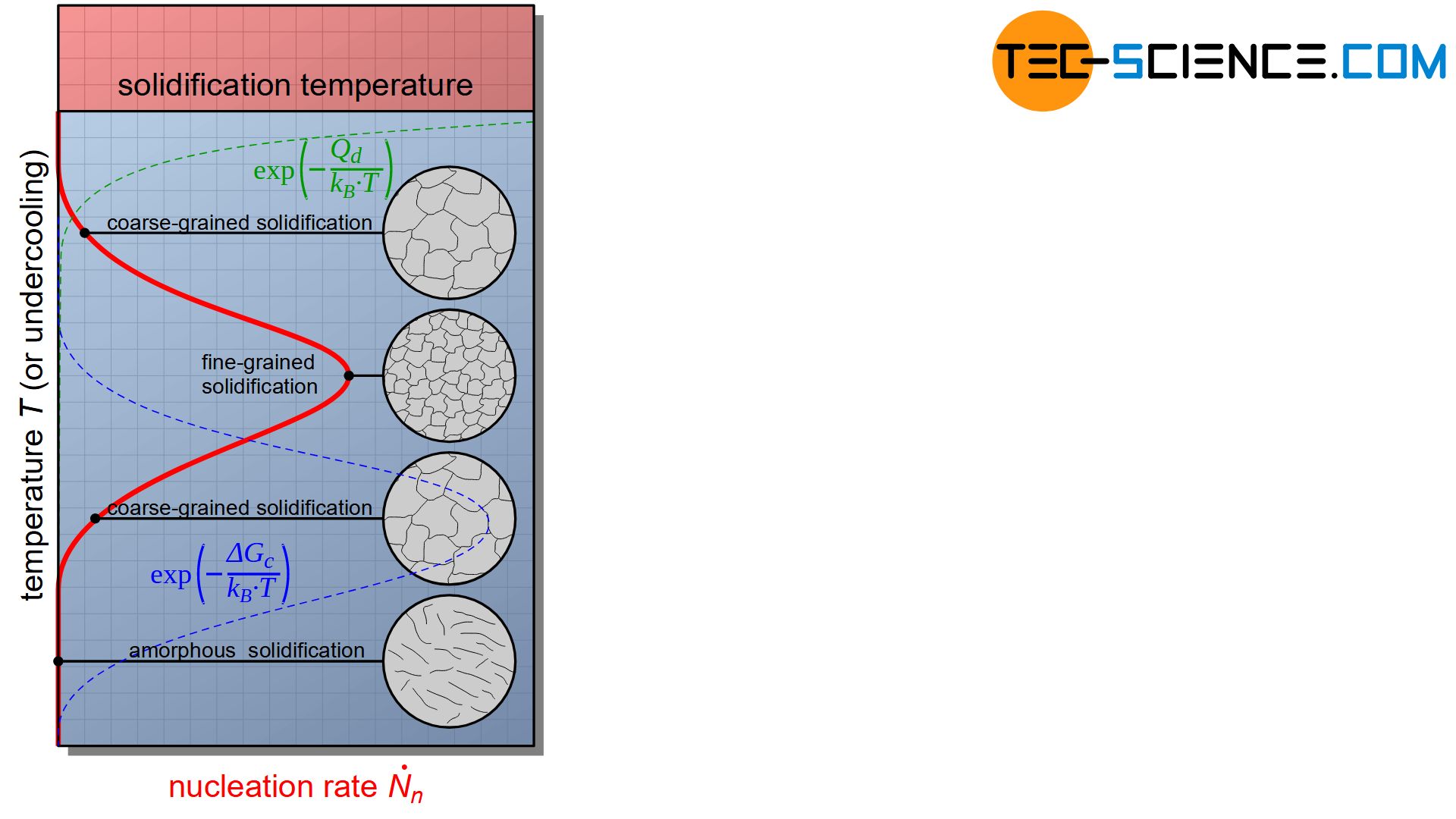



Homogeneous Nucleation Tec Science




Differences Between Homogeneous Nucleation And Heterogeneous Nucleation Rheingau Com




Food Freezing Homogeneous Nucleation Youtube




Thermodynamic And Kinetics Investigation Of Homogeneous And Heterogeneous Nucleation




Illustration Of Homogeneous Nucleation And Heterogeneous Nucleation Download Scientific Diagram




Difference Between Homogeneous And Heterogeneous Nucleation Compare The Difference Between Similar Terms



Pubs Acs Org Doi Pdf 10 1021 Jpf
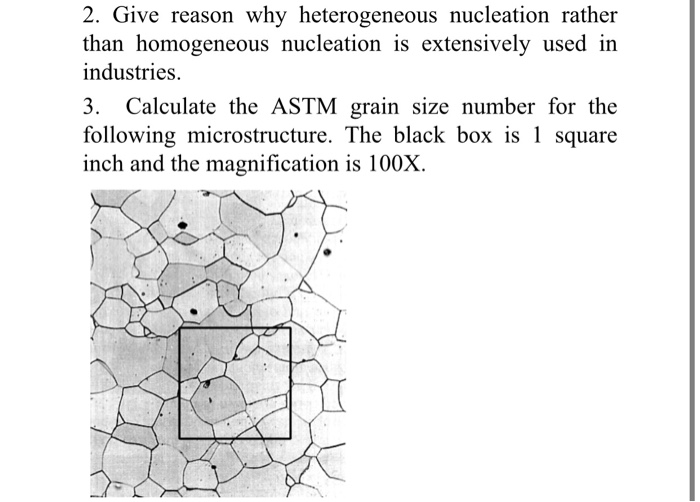



2 Give Reason Why Heterogeneous Nucleation Rather Chegg Com




Difference Between Homogeneous And Heterogeneous Nucleation Compare The Difference Between Similar Terms




Homogeneous Nucleation
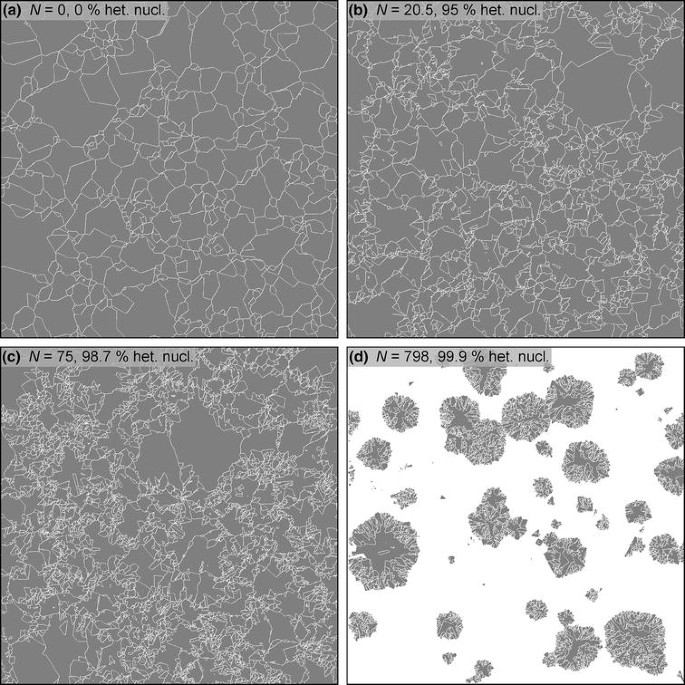



Heterogeneous Nucleation As The Predominant Mode Of Crystallization In Natural Magmas Numerical Model And Implications For Crystal Melt Interaction Springerlink



Kit Imk f Competences Aida Facilities




Quantitative Evaluation On The Heterogeneous Nucleation Of Amino Acid By A Thermodynamic Analysis Sciencedirect
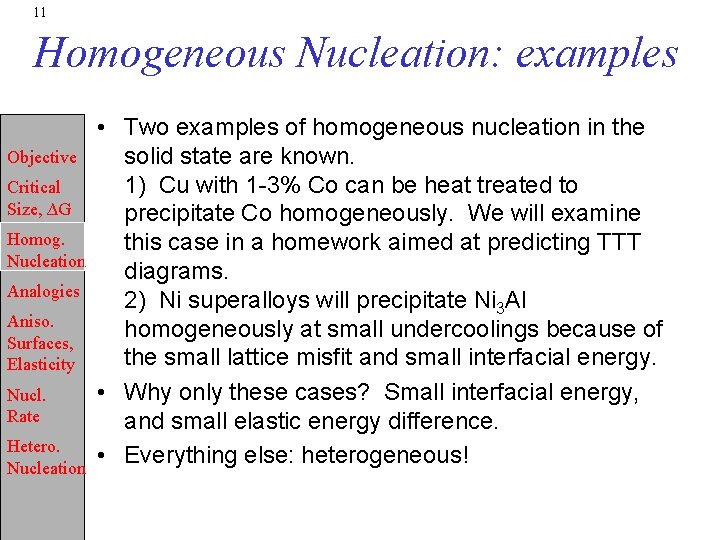



1 Objective Critical Size G Homog Nucleation Microstructureproperties
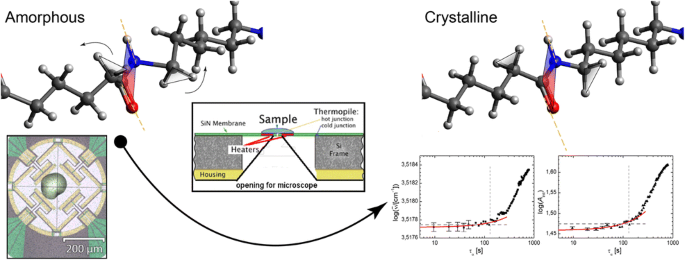



Fingerprints Of Homogeneous Nucleation And Crystal Growth In Polyamide 66 As Studied By Combined Infrared Spectroscopy And Fast Scanning Chip Calorimetry Springerlink



1




Difference Between Homogeneous And Heterogeneous Material Youtube



Chapter 10 Phase Transformations In Metals 1 Ppt Video Online Download
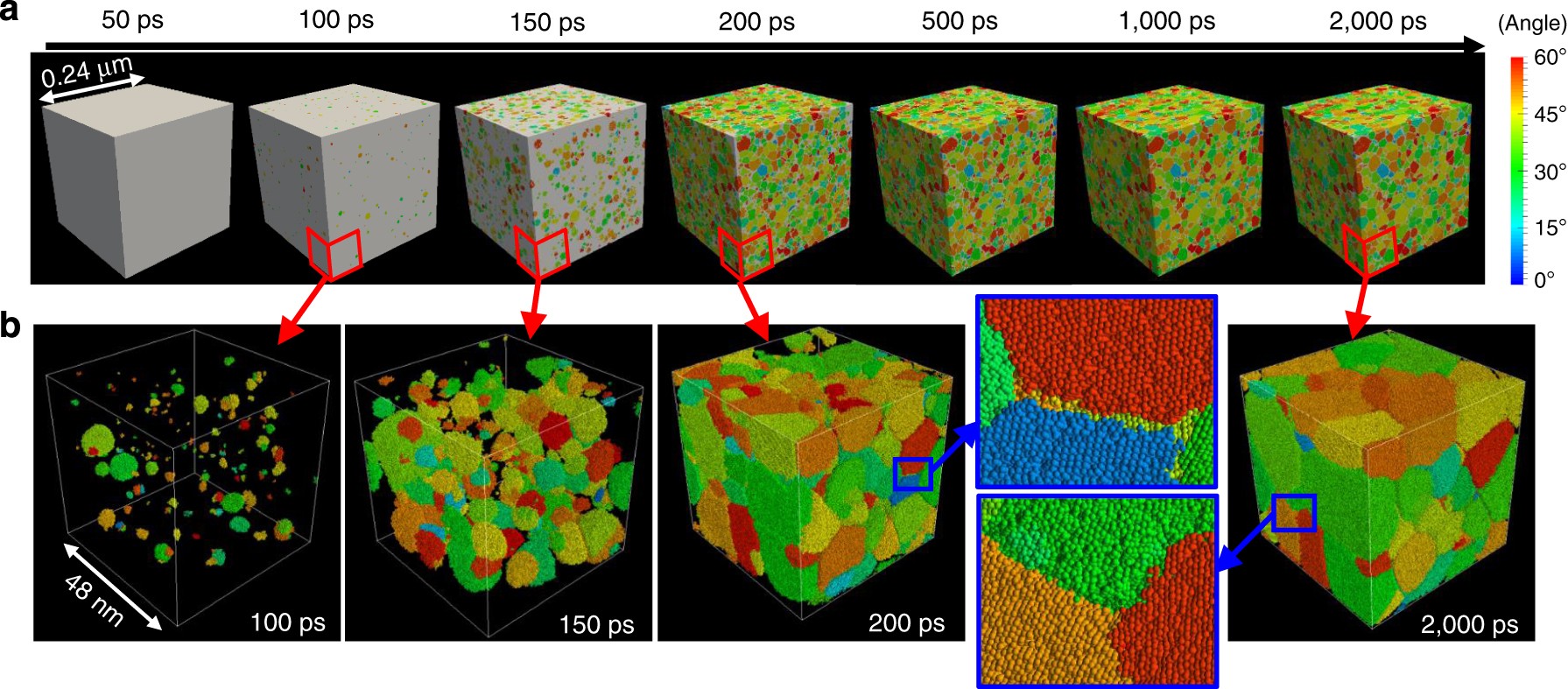



Heterogeneity In Homogeneous Nucleation From Billion Atom Molecular Dynamics Simulation Of Solidification Of Pure Metal Nature Communications




Schematic Representation Of Homogeneous And Heterogeneous Nucleation 44 Download Scientific Diagram




B Amyloid Aggregation And Heterogeneous Nucleation Srivastava 19 Protein Science Wiley Online Library




Homogeneous Nucleation




Organic Mineral Interfacial Chemistry Drives Heterogeneous Nucleation Of Sr Rich Ba X Sr1 X So4 From Undersaturated Solution Abstract Europe Pmc



1
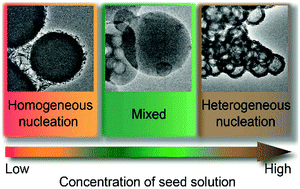



Heterogeneous Nucleation And Growth Of Highly Crystalline Imine Linked Covalent Organic Frameworks Chemical Communications Rsc Publishing
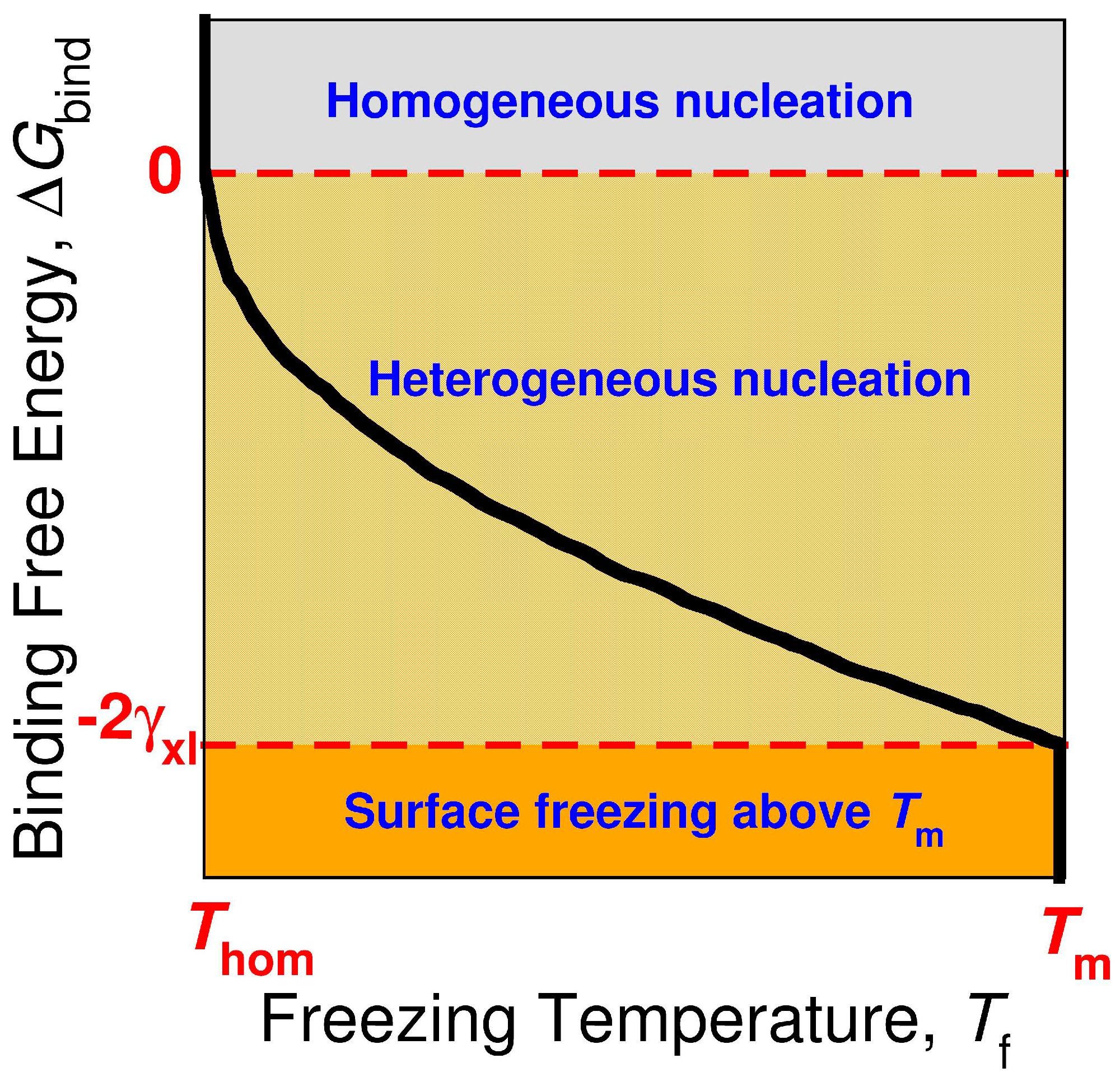



Crystals Free Full Text Strength Of Alkane Fluid Attraction Determines The Interfacial Orientation Of Liquid Alkanes And Their Crystallization Through Heterogeneous Or Homogeneous Mechanisms Html
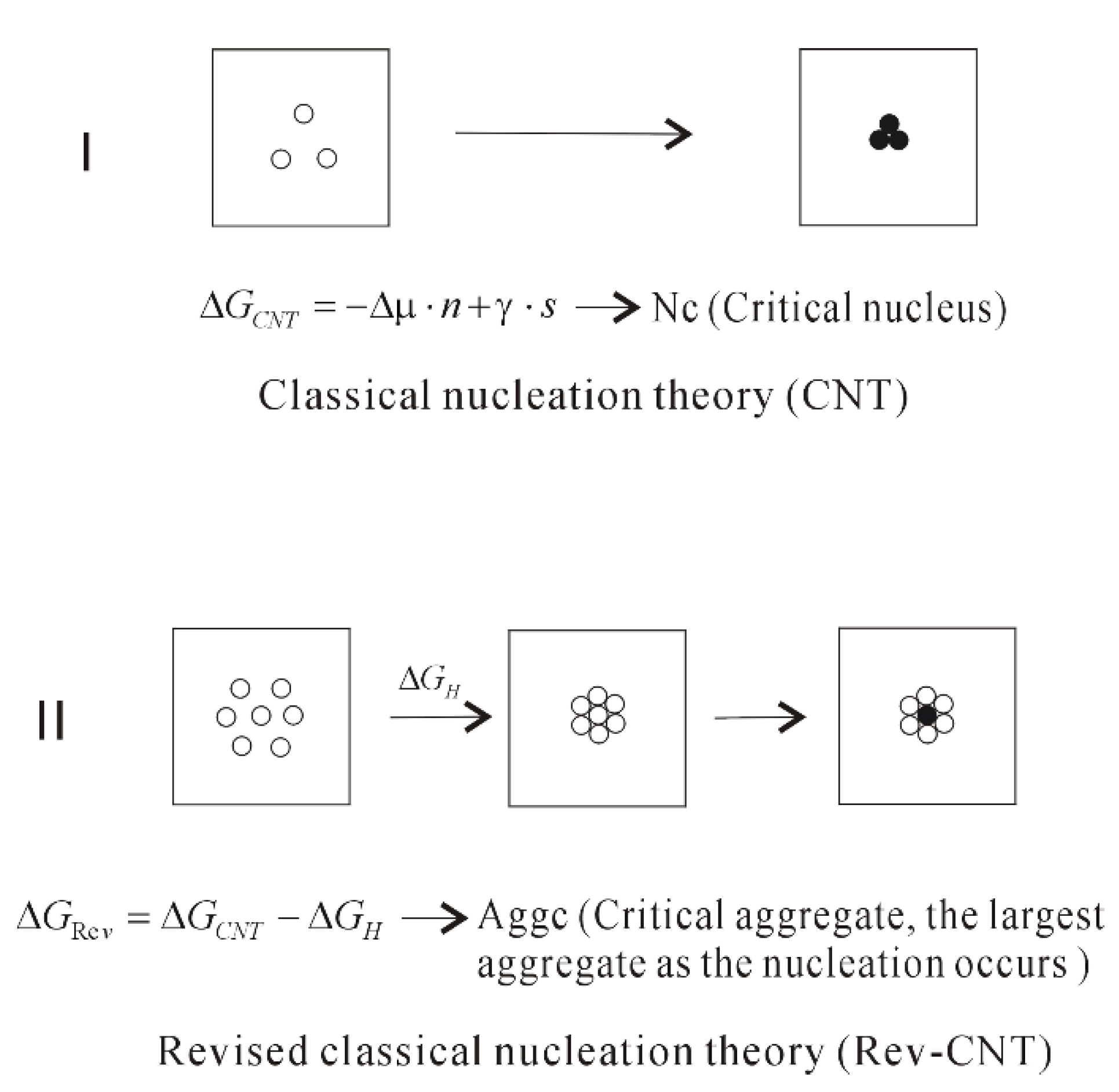



Crystals Free Full Text Homogeneous Nucleation Mechanism Of Nacl In Aqueous Solutions Html




Nanohub Org Courses Nanohub U Physics Of Electronic Polymers Spring 17




08 Homo Hetero Nucleation 1 Homogeneous Nucleation This Section Deals With The Simplest Nucleation Event Namely The Homogeneous Nucleation Of Solid Course Hero



Crystal Nucleation Chapter 3 Handbook Of Industrial Crystallization




Difference Between Nucleation And Particle Growth Compare The Difference Between Similar Terms




Organic Mineral Interfacial Chemistry Drives Heterogeneous Nucleation Of Sr Rich Bax Sr1 X So4 From Undersaturated Solution Pnas




Nucleation Intechopen



Pubs Acs Org Doi Pdf 10 1021 Acs Accounts 6b008
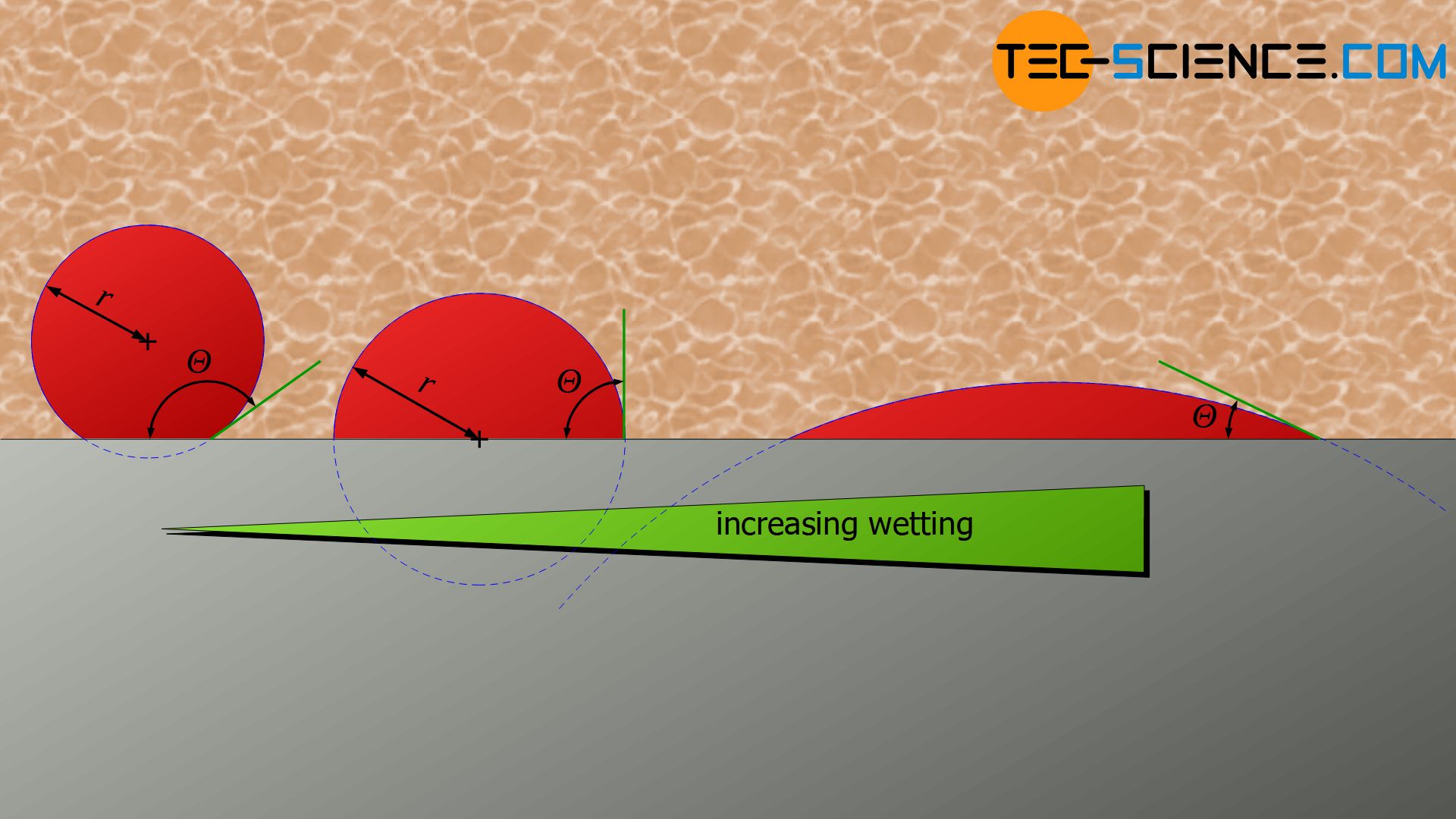



Heterogeneous Nucleation Tec Science




Homogeneous Nucleation
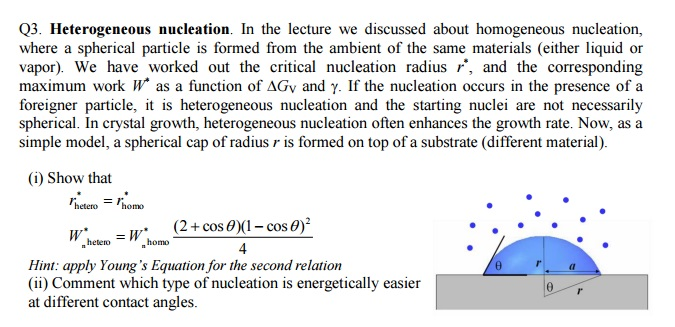



In The Lecture We Discussed About Homogeneous Chegg Com




5 Points In The Solidification Of A Pure Metal Chegg Com




Heterogeneous Nucleation And Crowding In Sickle Hemoglobin An Analytic Approach Biophysical Journal




Focus Article Theoretical Aspects Of Vapor Gas Nucleation At Structured Surfaces The Journal Of Chemical Physics Vol 145 No 21




Phase Transformation Nucleation 1 Of 5 Learning Chegg Com



Www Lehigh Edu Imi Teched Opg Lecture3 Pdf




Homogeneous And Heterogeneous Dislocation Nucleation In Diamond Sciencedirect




Schematic Representation Of Homogeneous And Heterogeneous Nucleation 44 Download Scientific Diagram
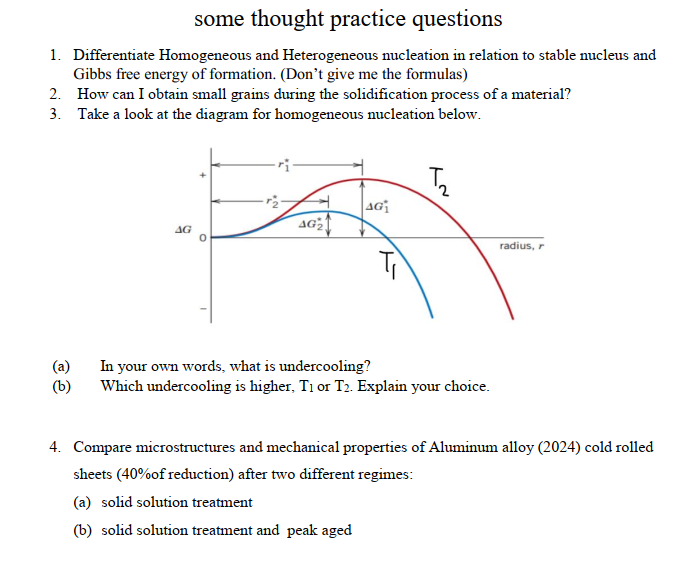



Some Thought Practice Questions 1 Differentiate Chegg Com




Scheme To Interpret The Change From Heterogeneous To Homogeneous Download Scientific Diagram




2 12 Homogeneous And Heterogeneous Nucleation Kinetics Of Structural Transformations Coursera


